Error tolerant search
Introduction
When the results of an MS/MS Ions Search of an LC-MS/MS dataset are reviewed, there will often be a number of spectra that remain unmatched. Assuming that a given MS/MS spectrum contains adequate information, i.e. a reasonable number of fragment ion peaks at usable signal to noise, possible reasons for this failure include:
- Underestimated mass measurement error
- Incorrect determination of precursor charge
- Enzyme non-specificity
- Unsuspected chemical & post-translational modifications
- Peptide sequence not in the database
If mass measurement error has been underestimated, this should be apparent from the graphs showing the differences between the calculated and measured mass values in the Peptide View and Protein View reports.
Incorrect determination of precursor charge has to be dealt with during peak detection. If it is not possible to determine the precursor charge reliably, then one option is to generate peak lists for all probable charge states.
The Mascot Error Tolerant Search addresses the final three difficulties by searching selected database entries with relaxed enzyme specificity, while iterating through a comprehensive list of chemical and post-translational modifications, together with a residue substitution matrix.
There are two ways to perform an error tolerant search. The preferred method is to check the error tolerant checkbox on the search form, which leads to an automatic, second pass search. There is also a manual procedure, in which the user selects the proteins that will go forward for the second pass search. This was an earlier implementation, and is retained mainly for compatibility with existing workflows and third party software.
Note that both methods are only applicable to MS/MS data; it is not possible to perform an error tolerant peptide mass fingerprint. For a truly unknown modification, or a sequence variation of more than a single base or residue, the error tolerant sequence tag is worth investigating.
Automatic Error Tolerant Search
An automatic error tolerant search is performed by choosing the error tolerant checkbox on the search form. A standard, first pass search is performed using the search parameters specified in the form. From the results of the first pass search, all of the database entries that contain one or more peptide matches with scores at or above the homology threshold, (or identity threshold if there is no homology threshold), are selected for an error tolerant, second pass search. At the completion of the second pass search, as single report is generated, combining the results from both passes.
During the error tolerant, second pass search:
- The selected enzyme becomes semi-specific, (that is, only one end of a peptide needs to match the cleavage specificity), and the value of the missed cleavage parameter is increased by 1
- The complete list of modifications is tested, serially
- For a protein, the set of all possible amino acid substitutions is tested. For a nucleic acid sequence, all single base insertions, deletions, and substitutions are tested.
- Only one of the above is allowed per peptide. That is, an individual peptide can be semi-specific OR have one unsuspected modification OR have one primary sequence mutation.
- If the mass delta of the modification is less than the smaller of the precursor mass tolerance and the fragment mass tolerance, the modification is rejected. This eliminates modifications that are meaningless given the estimated mass error, like Q->K, in most cases.
The following constraints apply to the standard, first pass search:
- Enzyme must be fully specific
- A reduced ceiling on the number of variable modifications, (default is 2, but this can be changed globally in mascot.dat or for a user group in Mascot security)
- Cannot be combined with an automatic decoy database search
- Cannot be combined with quantitation
- Search cannot include error tolerant sequence tag
Manual Error Tolerant Search
Database entries are selected from the results report of a standard search. Check the Error tolerant checkbox, near the Search selected button, and choose one or more proteins to be included in the search. (On the public web site, a maximum of 3 proteins can be chosen). Clicking on the Search selected button loads a modified search form, from which you can change many of the search parameters. Cleavage agent defaults to None, though an enzyme can be chosen if desired.
During the error tolerant search:
- The complete list of modifications is tested, serially
- For a protein, the set of all possible amino acid substitutions is tested. For a nucleic acid sequence, all single base insertions, deletions, and substitutions are tested.
The manual error tolerant search should only be used in exceptional cases. One reason is that, because enzyme specificity is dropped entirely, and modifications can be combined with non-specificity, and the number of database entries tends to be fewer, the level of "junk" matches in the manual search will be higher than in the automatic search. Another reason is that, in the automatic search, the results from both passes are saved to the result file, which provides greater reporting flexibility. For example, you can choose to show or hide the additional, error tolerant matches. The combined report also reduces compatibility problems for applications that read Mascot result files.
Reviewing the Results
It is important to recognise that only the matches from the standard, first pass search provide evidence for the identity of a protein. The additional matches found in the error tolerant, second pass search are valuable because they are the most likely assignments of the spectra. Occasionally, an additional match will provide useful biological information, such as distinguishing between two isoforms. If the same modification shows up many times, this may indicate an experimental artefact that needs to be eliminated or, at least, selected as a variable modification for standard searches.
Nevertheless, these additional matches have been obtained by selecting a small number of database entries and beating them into submission with non-specificity, substitutions and a long list of modifications. This makes it difficult to apply any meaningful measure of statistical significance. So, in the result report, the error tolerant matches are treated differently from the standard matches:
- They do not contribute to the protein score. (If the query also has a lower scoring match to the same protein in the first pass search, this contributes, so that the protein scores are identical to those seen in the standard report.
- Significance thresholds are not reported
- Expect values are not reported
- Must have scores of at least the identity threshold for the query in the first pass search
- Must have scores in excess of the highest scoring match to the query in the first pass search
(Item 1 only applies to the combined report, obtained from an automatic error tolerant search. If you perform a manual error tolerant search, the report will show protein scores derived from all the matches listed.)
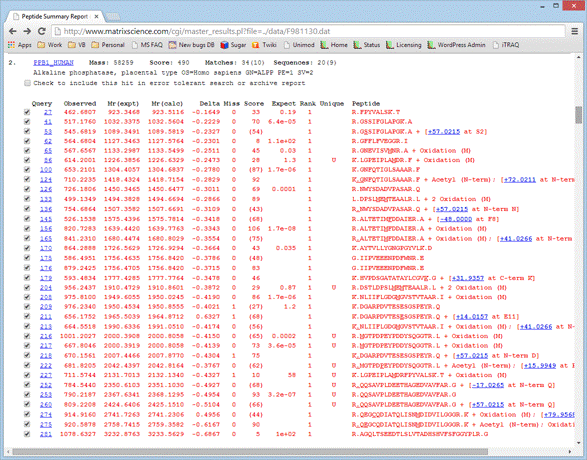
For example, click on this thumbnail image to load an example of the results from an error tolerant search in a new browser window. Scroll down to hit 2, Alkaline phosphatase.
The additional. error tolerant matches can be recognised easily as the ones with gaps in the expect value column. In some cases, the additional match is the result of non-specific cleavage, such as queries 133 and 176. If the error tolerant match was found by introducing a modification or a sequence change, the mass delta and its location are given at the end of the row. When the mouse rests over the mass delta hyperlink, all the known assignments of this delta are displayed in a pop-up. Take a look at query 218. The mass tolerance for this search was fairly wide, ±0.8 Da, so the observed mass difference could correspond to either carbamidomethylation or carboxymethylation at the N-terminus. Since this sample was alkylated with iodoacetamide, we would choose carbamidomethylation as the more likely suspect, especially as this brings the error on the precursor mass into line with the general trend, whereas carboxymethylation would give an error of +0.6 Da. The assignment to carbamidomethylation is also very believable, because this is a known artefact of over-alkylation. The same modification is found for queries 53 and 260.
Another easily believable assignment is pyro-Glu for the match to query 252. In other cases, the match may be good, but the assignment is not believable. For example, look at query 145, which has a mass difference of -48.0 Da, assigned to a substitution, F->V. This is not feasible because the native peptide is matched strongly by the next two queries. Figuring out a reasonable assignment for some matches can require a bit of detective work. In this case, notice that the next two matches show the Met as being oxidised. If we hypothesise that the Met in the match to query 145 is also oxidised, this would take the mass difference to -64 Da. It is well known that oxidised Met loses 64 Da, (methanesulfenic acid), during CID. The most likely explanation for this match is that there was prompt loss of 64 Da from oxidised Met, possibly due to in-source collision.
Always check the alternative matches that are displayed when the mouse cursor rests over the query number or by clicking on the query number to load a Peptide View report. It is common to get multiple matches with very similar scores. The best match may be a very unlikely modification, while a match with a lower score has a more credible explanation.
More about Modifications
The list of modifications used by Mascot is taken directly from the Unimod database. For further details of individual modifications, please refer to Unimod.
Note that only a small sub-set of modifications is displayed by default in the Mascot search form. If you want to see the complete list, you must go to the search form defaults page and tick the checkbox for Show all mods.
In an Error Tolerant search, all the entries on the modifications list are tested serially, and all permutations of each individual modification are tested. For example, if a modification affects serine, and a peptide contains three serines, but has a molecular mass consistent with just two modifications, there are 3 permutations to be tested (110,101,011).
This differs from the behaviour for any variable modifications explicitly specified in the search form, when all permutations and combinations of the selected modifications are tested. Specifying more than a handful of variable modifications leads to a drastic loss of discrimination, because the number of permutations and combinations increases geometrically with the total fractional abundance of modifiable residues.
More about Sequence Variants
Variations in the primary sequence generally result from variations in the DNA sequence. These may be DNA sequencing errors, they may be mutations or polymorphisms, or they may be more extensive evolutionary changes, because the database entry is not the authentic protein, but a related sequence from a different species.
When searching a nucleic acid database, single base deletions and insertions can be tested in addition to substitutions. The consequences of deletions and insertions cannot be tested for a protein database because they cause a frame shift, which completely changes the amino acid sequence from that point onwards.
Amino acid substitutions in protein sequences are handled like modifications, and the composition and mass changes are taken from Unimod entries.